Creating Clarity
We help pets lead fuller lives by giving veterinarians the tools, technology, and insights to see clearly and get the answers they need.


- Online Test Directory
- IDEXX Online Orders
- IDEXX Learning Center

How we create clarity
Here are just a few ways we’re helping veterinary professionals practice at their best.
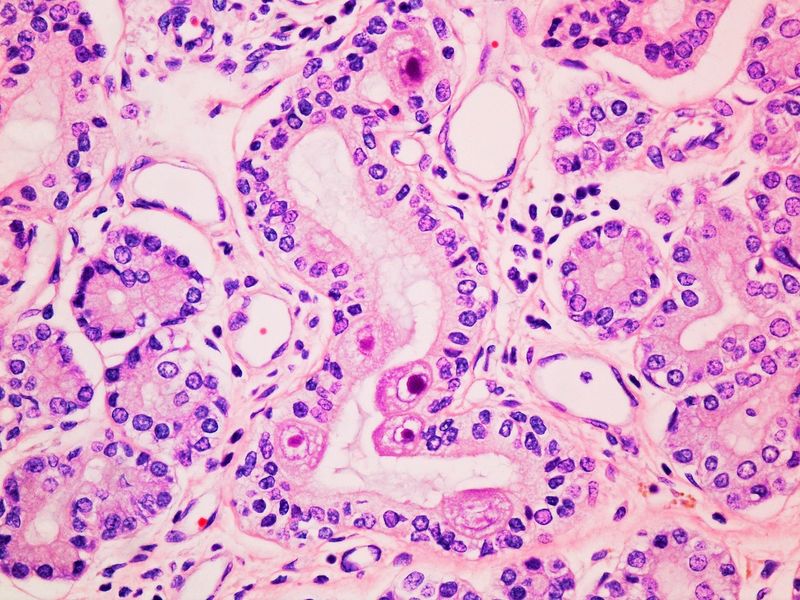
IDEXX Reference Laboratories
An extension of your practice so you can focus on your patients.
Point-of-care
Deep, actionable insights while your patients are on-site.
Practice management
Taking care of your practice
—from software to CE.

See cells like never before
Diagnose and act on clinically important cell types with reference laboratory-level accuracy, in real time, with a slide-free workflow.
IDEXX Learning Center
Everything you need to stay ahead of your CE and then some. All for free.
- RACE-accredited courses
- Webinars and live events
- Learn seamlessly across any device






IDEXX Stories
From our innovations to our people.
From our innovations to our people—here are some of the ways we’re proud to have a positive impact on our industry, community, and planet.